
DESIGN AND DEVELOPMENT FROM A TO Z
FINISHED PRODUCT AT YOUR FINGERTIPS
GOFARM WILL PROVIDE YOU WITH A FULL RANGE OF SUPPORT SERVICES
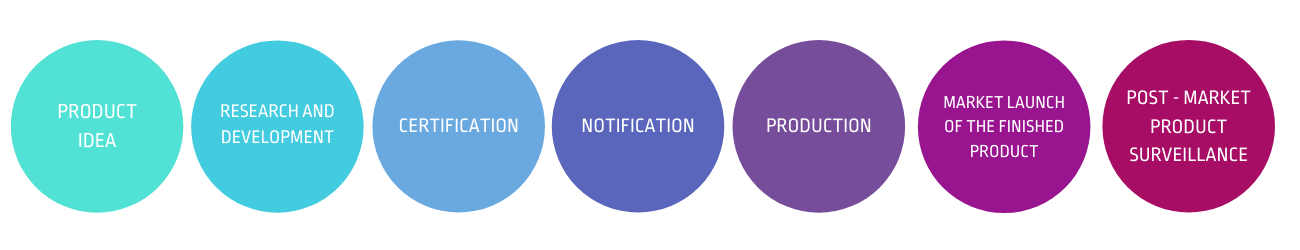
FROM PRODUCT IDEA, RESEARCH AND DEVELOPMENT, PRODUCT CERTIFICATION, NOTIFICATION, PRODUCTION,
TO MARKET LAUNCH OF THE FINISHED PRODUCT,
AS WELL AS POST-MARKET PRODUCT SURVEILLANCE.
We offer the first comprehensive R&D service for pharmaceutical medical devices in Poland,
i.e. from an idea to a finished product on the shelf!
With us, you can easily launch a new product on the market or adapt an existing one to new legal requirements.
Gofarm’s R&D Team
has developed and launched
more than 50 medical devices
on the EU and non-EU markets
over the past 10 years
for around 150 private label brands.
We have issued dozens of opinions on the composition of planned medical devices and full technical documentation for Polish pharmaceutical companies.
Design and development of medical devices is carried out by an experienced and qualified R&D Team cooperating with national and international notified bodies.
WE OFFER
A COMPREHENSIVE MEDICAL DEVICE DEVELOPMENT SERVICE
IN A SCOPE CHOSEN BY YOU
TRUST THE EXPERIENCE AND KNOWLEDGE OF EXPERTS WHO HAVE SUCCESSFULLY LAUNCHED MANY PRODUCTS FOR THEIR CUSTOMERS
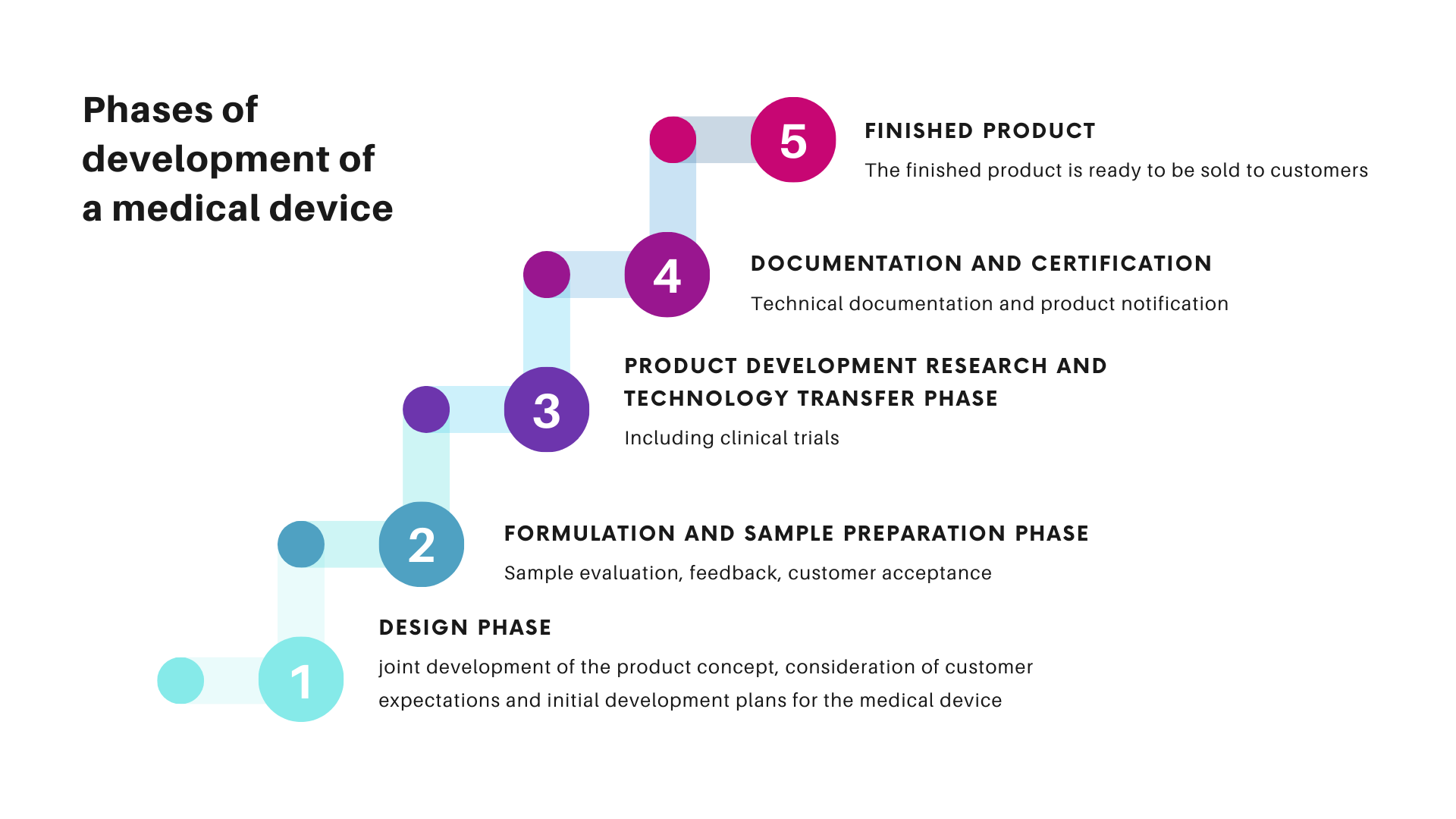
- Designing and development of medical devices in compliance with MDR requirements and Customer’s assumptions
- Modification of existing formulas of medical devices in order to adjust them to the new MDR requirements
- Preparing opinions on compliance of ingredients/concepts of medical devices with MDR requirements
- Necessary examinations of product designs
- Introduction of prepared concepts to production
- Support for clinical trials of medical devices
- Assistance in the process of certification of medical devices by a notified body
- Provision of a person responsible for regulatory compliance
The aforementioned services are conducted on the basis of the following legal basis: Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC

